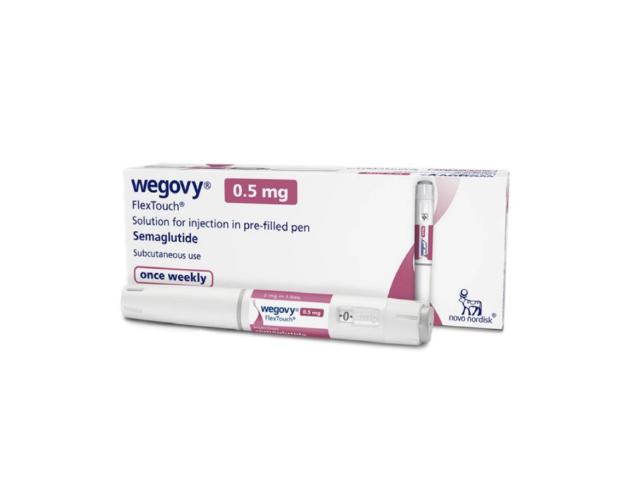
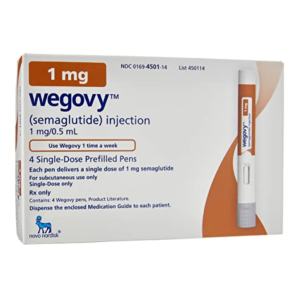
Wegovy® (semaglutide) 4 pens | 0.5 mg
£1,349.00
Wegovy® (semaglutide) 0.5 mg weekly injection is a prescription drug approved by the FDA to help adults who are overweight or obese and have health problems linked to their weight. It comes in 4 pre-filled pens that can be used for 28 days. When combined with diet and exercise, it helps control blood sugar, lower hunger, and make you feel full.
Wegovy® (Semaglutide) 0.5 mg Weekly Injection – 4 Pens
FDA-Approved Prescription for Chronic Weight Management
Wegovy 0.5 mg is an FDA-approved prescription injectable medication indicated for chronic weight management in adults with obesity or overweight who have at least one weight-related health condition, including type 2 diabetes, high blood pressure, or high cholesterol. It is clinically proven to support significant and sustainable weight loss when combined with lifestyle changes.
GLP-1 Receptor Agonist That Targets Appetite Control
Each pre-filled pen delivers Wegovy 0.5 mg of semaglutide, a GLP-1 receptor agonist that works by mimicking natural hormones involved in appetite and glucose regulation. Wegovy® helps:
- Reduce appetite and food cravings
- Increase feelings of fullness after meals
- Support healthy blood sugar regulation
This mechanism supports improved portion control and long-term weight management.
Convenient Once-Weekly Subcutaneous Injection
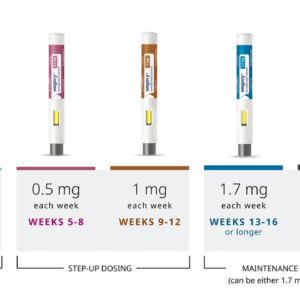
Wegovy® is administered once weekly via subcutaneous injection, offering a simple and convenient dosing schedule. The 0.5 mg strength is commonly used as part of a gradual dose-escalation plan to help the body adjust to treatment and minimize gastrointestinal side effects.
Best Results With Diet and Physical Activity
For optimal outcomes, Wegovy® should be used alongside a reduced-calorie diet and increased physical activity. This combined approach enhances weight-loss results and supports overall metabolic health.
Product Details
- Medication Name: Wegovy® (semaglutide)
- Dosage Form: Pre-filled injection pens
- Strength: 0.5 mg per pen
- Quantity: 4 pens
- Dosing Schedule: Once weekly
- Typical Supply: 28-day supply
Use Under Medical Supervision
Wegovy® is available by prescription only and should be used under the supervision of a licensed healthcare provider. Proper dose escalation is important to reduce potential gastrointestinal side effects and to maximize the safety and effectiveness of weight-loss treatment.
Be the first to review “Wegovy® (semaglutide) 4 pens | 0.5 mg” Cancel reply
Related products
Wegovy
Wegovy




Reviews
There are no reviews yet.